Request Appointment
Enter your details and we will be in touch with you shortly;
Or call
8655885566
between 8 am and 8 pm.

Low back pain is a common cause of disability in people over 65 years of age, and almost 70% of elderly patients with musculoskeletal problems have LBP [1–3]. With the increase in elderly population all over the world, the burden of disability due to LBP is expected to rise in the coming decades [4]. Although disability from LBP is the highest in working age groups worldwide, 1-year prevalence of LBP in subjects over 65 years of age can vary across the world and is reported to be as high as 50% [3, 5–8]. Older patients are more prone to experience severe LBP [9], more prone to develop chronic LBP [6, 10] and have poorer treatment outcomes despite the use of interventional surgical techniques [11, 12]. Furthermore, older adults with chronic LBP may have a worse prognosis due to increased risk of falling due to LBP [13], increased risk of mortality with the presence of LBP [14] and the presence of more psychosocial dysfunction and physical performance impairments in the presence of LBP [15].
Although the frst line of treatment for chronic LBP in patients over 65 years of age continues to be conservative management (including exercise therapy), there is not enough evidence supporting any particular rehabilitation program for back pain in older patients and the usual practice is to treat these patients using protocols commonly used for younger population with chronic LBP [5, 16]. Nascimento et al. [17] in a recent meta-analysis on the efectiveness of interventions for non-specifc LBP in older adults (≥60 years) reported that evidence about interventions to manage non-specifc LBP in older adults is weak and most clinical trials for older adults were usually based on passive interventions.
We have used an integrated active, rehabilitation protocol for patients≥65 years of age presenting with chronic LBP with the aim to improve clinical outcomes in this patient age group. Hence, the aim of the current retrospective study was to determine the efcacy and clinical outcome of an integrated active, rehabilitation protocol in patients≥65 years of age with chronic LBP and compare the results of this treatment in similar patients in the 50–64 years of age group with chronic LBP and to determine the factors which afect clinical outcome in patients≥65 years of age. We hypothesised that clinical outcomes with an integrated active, rehabilitation protocol for patients≥65 years of age presenting with chronic LBP will be similar to outcomes in 50–64 years patient age group.
We retrospectively reviewed electronic records of 17,921 patients treated conservatively with an active rehabilitation protocol for low back pain (LBP) at a chain of centres specialising in spine rehabilitation from November 2016 to December 2018. The inclusion criteria were patients with chronic mechanical LBP who underwent treatment at our centres in one of the two age groups of 50–64 years and≥65 years. The older or “elderly” age group was defned as patients with chronological age of 65 years or above based on clinical practice guidelines previously published in the literature [18, 19]. The exclusion criterion were patients with red fags conditions such as infection, tumour and fractures: patients with infammatory conditions such as rheumatoid arthritis and spondyloarthropathies, patients with involvement of peripheral joints such as hip, knee and ankle joints, patients with structural kyphotic or scoliotic deformities, patients with peripheral neuropathy, patients with lumbar canal stenosis (diagnosed clinically based on the presence of neurogenic claudication and fndings of central canal stenosis on magnetic resonance imaging) and spondylolisthesis, patients with complex pain syndrome, patients with incomplete clinical records and patients who did physiotherapy for less than 1 month or more than 3 months at the centre. Mechanical low back pain was defned as low back pain arising intrinsically from the spine, intervertebral discs or surrounding soft tissues which may worsen with specifc spine movement and often improves with rest [20]. Non-mechanical low back pain was defned as low back pain arising due to an underlying infammatory condition such as rheumatoid arthritis or spondyloarthropathies or secondary to any red fag condition such as infection, tumour and fractures [20].
All patients were evaluated before and during the course of treatment for their LBP. Demographic data including gender, age, body mass index (BMI), lifestyle and smoking were collected from all patients. Lifestyle was categorised as sedentary, semi-active and active. Sedentary lifestyle was defned as prolonged sitting at work, during leisure time and lack of physical activity/exercise or movement during most part of the day; semi-active lifestyle was defned as lifestyle more active than sedentary but not performing daily moderate-tovigorous physical activities; and active lifestyle was defned as performing daily moderate-to-vigorous physical activities as per the American College of Sports Medicine (ACSM) recommendations [21–23].
A thorough history of presenting complaints, past illness, previous surgical or non-surgical treatment or any red fag conditions (recent trauma, night or at rest pain, fever, unexplained weight loss, progressive motor or sensory defcit, bowel or bladder symptoms, history of cancer, chronic steroid use and immunosuppression) was recorded. Red fag conditions and non-mechanical causes of LBP were ruled out based on history, clinical examination and radiographic
and blood reports. All patients were clinically examined for posture, lumbar spine range of movement (forward fexion, extension and lateral fexion) and motor and sensory function (myotomal and dermatomal loss) by a physiotherapist in the clinic. Pain before and after treatment was recorded using the numerical pain rating scale (NPRS) with pain intensity ranging from “0” (no pain) to “10” (worst pain imaginable) [24]. Functional disability before and after treatment was recorded using the Oswestry disability index (ODI) score [25]. All patients were also evaluated and diagnosed based on the mechanical diagnosis and therapy (MDT) as reducible or irreducible derangement, postural syndrome, dysfunction or other (to included spondylolisthesis and stenosis) by a senior physiotherapist in the clinic certifed in the MDT method [26].
An integrated active, rehabilitation protocol primarily based on severity of pain on NPRS, limitation in activities of daily
living (ADL) or the presence of factors acting as “barriers to recovery” and movement testing was administered to patients≥65 years of age patients undergoing treatment for chronic mechanical LBP (Fig. 1). This rehabilitation protocol was similar to the protocol for treating patients in the 50–64 years age group and involved a combination of functional training wherever required, patient education on posture, frequency-specifc microcurrent (FSM) and directional movement based on MDT to reduce pain, strength training on a device and stabilisation exercises. Treatment in all patients was performed at an outpatient clinic.
All patients were frst thoroughly assessed through history-taking, clinical examination and investigation reports to rule out the presence of red fag conditions and to determine whether the cause of chronic LBP was mechanical or nonmechanical. Patients identifed as having red fag conditions or non-mechanical causes of chronic LBP were referred to a physician or an orthopaedic surgeon for further evaluation and treatment. The patients with mechanical chronic LBP were then evaluated for the presence of “barriers to
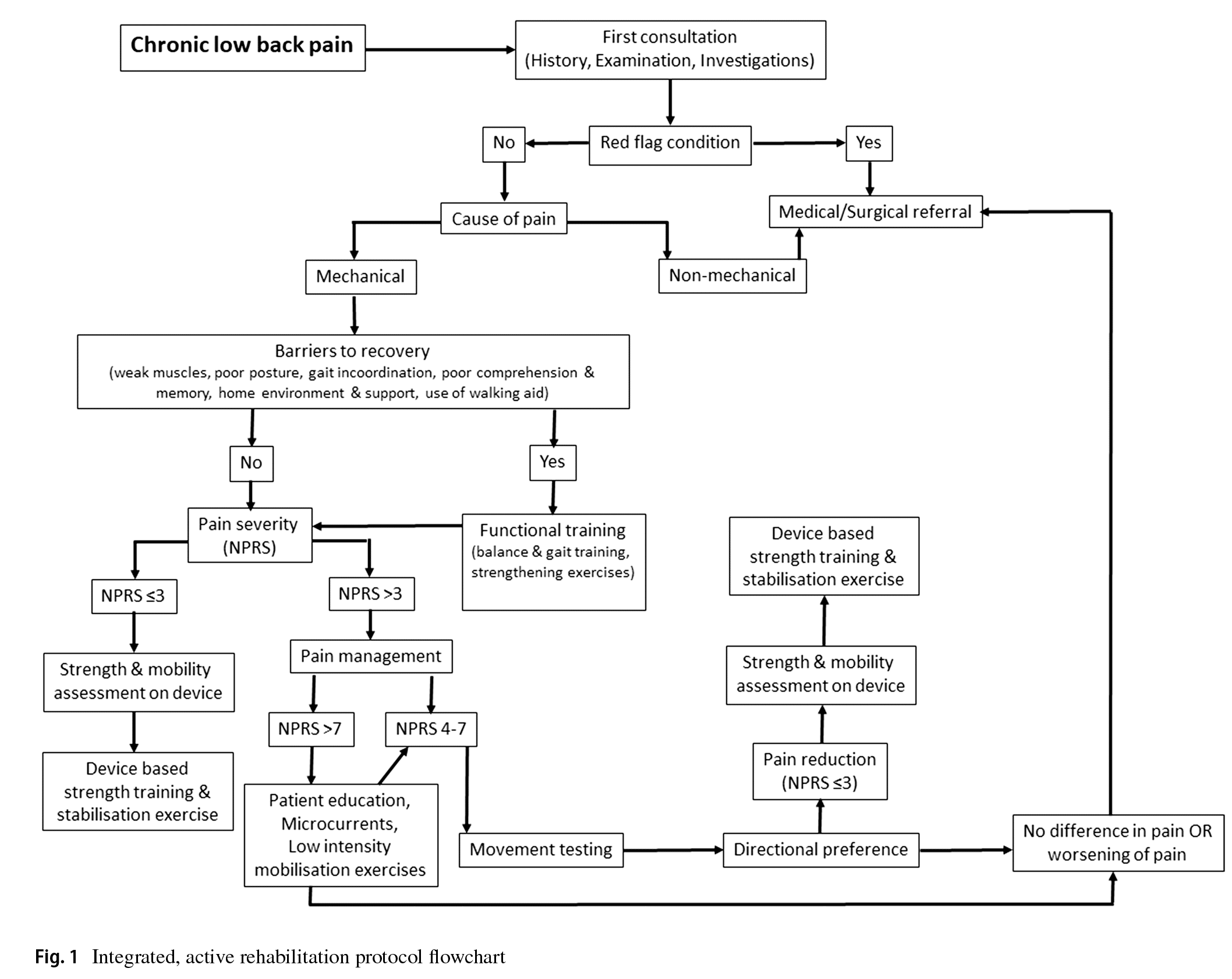
recovery” which may hinder their overall progress and outcome after the rehabilitation therapy. These barriers included factors such as weak muscles, poor posture, gait incoordination, poor comprehension and memory, home environment and support, use of a walking aid. Patients with such barriers were frst put through functional training which included balance and gait training and strengthening exercises and education and instruction about their condition for the initial 7–10 days after which their rehabilitation protocol was administered. Based on the severity of LBP on the NPRS score during the frst assessment, patients were advised FSM therapy, low intensity extension mobilisation exercises for muscle activation, patient education about maintaining proper posture and rest (breaking posture and going to a non-loading position like lying supine) when required during ADL for a maximum of 1 week if NPRS score was≥7. In such patients, once the pain on NPRS score was 4–7 or in patients presenting with pain on NPRS score 4–7, movement testing was done using the MDT method to determine directional preference [26]. The patients were then advised directional movements which was performed under supervision by the treating physiotherapist in the outpatient clinic and advised to continue these movements at home at least 4–5 times a day, 10 repetitions per session till NPRS score reduces to<4. Patients were educated on the concept of “peripheralisation” of symptoms (i.e. symptoms migrating away from the middle line of the body and towards the lower limb) and asked to discontinue any movement which caused peripheralisation of pain and consult their therapist for further advise [26]. The patients were reviewed on a weekly basis for progress or improvement in pain and function. Once the pain on NPRS score was<4, paraspinal muscle strength and lumbar spine mobility were assessed using the David Spine Concept system (David Health Solutions, Helsinki, Finland) device which objectively measured lumbar spine movement loss and fexibility and paraspinal muscle strength and endurance [27]. This device is equipped with a knee-lock system and a thigh restraining belt to immobilise both hips and thighs which allowed the patient to move only the lower back. The patient was then put on a customised strengthening and stabilisation exercise protocol based on the readings of the device. The program aimed to increase both strength and endurance of the back/paraspinal muscle with 15 to 20 repetitions every session on the device. Training load on the device was increased or decreased based on the patient’s ability to perform 20 repetitions of slow and controlled back extension and fexion movements [27]. For patients presenting with NPRS score was≤3, strength and mobility testing was directly performed on the device and customised strengthening and stabilisation exercise protocol administered based on device readings. The patients were then advised to perform these strengthening and stabilisation exercises at home at least two times a day.
All patients underwent a minimum rehabilitation treatment of 30 days and maximum of 90 days. A minimum of six supervised physiotherapy sessions at the clinic were advised to all patients in order to ensure patient compliance and accurate clinical review. Post-treatment clinical assessment was performed at the time of discharge to determine NPRS score, ODI score and disability category. Treatment outcome of a patient was considered a full success if the patient shifts to a disability category of “minimal” and pain NPRS score of≤1 and partial success if the patient improves in disability by one category (e.g. shift from severe to moderate category) and pain NPRS score of≤3 after treatment. Treatment outcome of a patient was considered a failure if there was no change in the degree of pain or disability at the end of treatment.
A post hoc power calculation using the primary variable post-treatment NPRS improvement percentage was performed. Setting the type-I error at 0.05, the power of the study was found to be 93.1. Demographic data including gender, age, body mass index (BMI), lifestyle, history of diabetes, osteoporosis, smoking and night pain, primary diagnosis on MDT assessment, response of pain to movement testing, treatment sessions done and total treatment period were analysed. Similarly, clinical outcome data such as NPRS score, percentage of NPRS score improvement, ODI score and treatment outcome category were analysed. A minimal clinically important diference (MCID) between pre- and post-treatment NPRS and ODI scores was used to compare and determine clinically signifcant improvement in pain intensity and disability. Based on recommended MCID thresholds for LBP patients in the literature, the threshold was set at 2 points improvement (on a scale of 0 to 10) for NPRS score and the threshold was set at 10 points improvement from pre- to post-treatment score (on a scale of 0 to 100) for ODI score [24, 28]. Pre and post-treatment clinical outcome parameters such as NPRS score, ODI score, percentage of NPRS score improvement, treatment outcome category and percentage of patients who achieved MCID thresholds for NPRS and ODI scores were compared between the 50–64 years age and≥65 years age groups to determine efectiveness of treatment. Categorical data were compared using the Fisher’s test, and continuous data were compared using the t test or chi-square test. A multivariate analysis was performed to determine the efect of independent variables such as age, gender, history of diabetes, osteoporosis or smoking, pre-treatment NPRS score and pretreatment ODI score on NPRS score, percentage of NPRS score improvement and ODI score after treatment in both the age groups. A p value of<0.05 was considered signifcant. Statistical analysis was performed using the GraphPad
QuickCalcs online statistical analysis tool (GraphPad Software, San Diego USA)
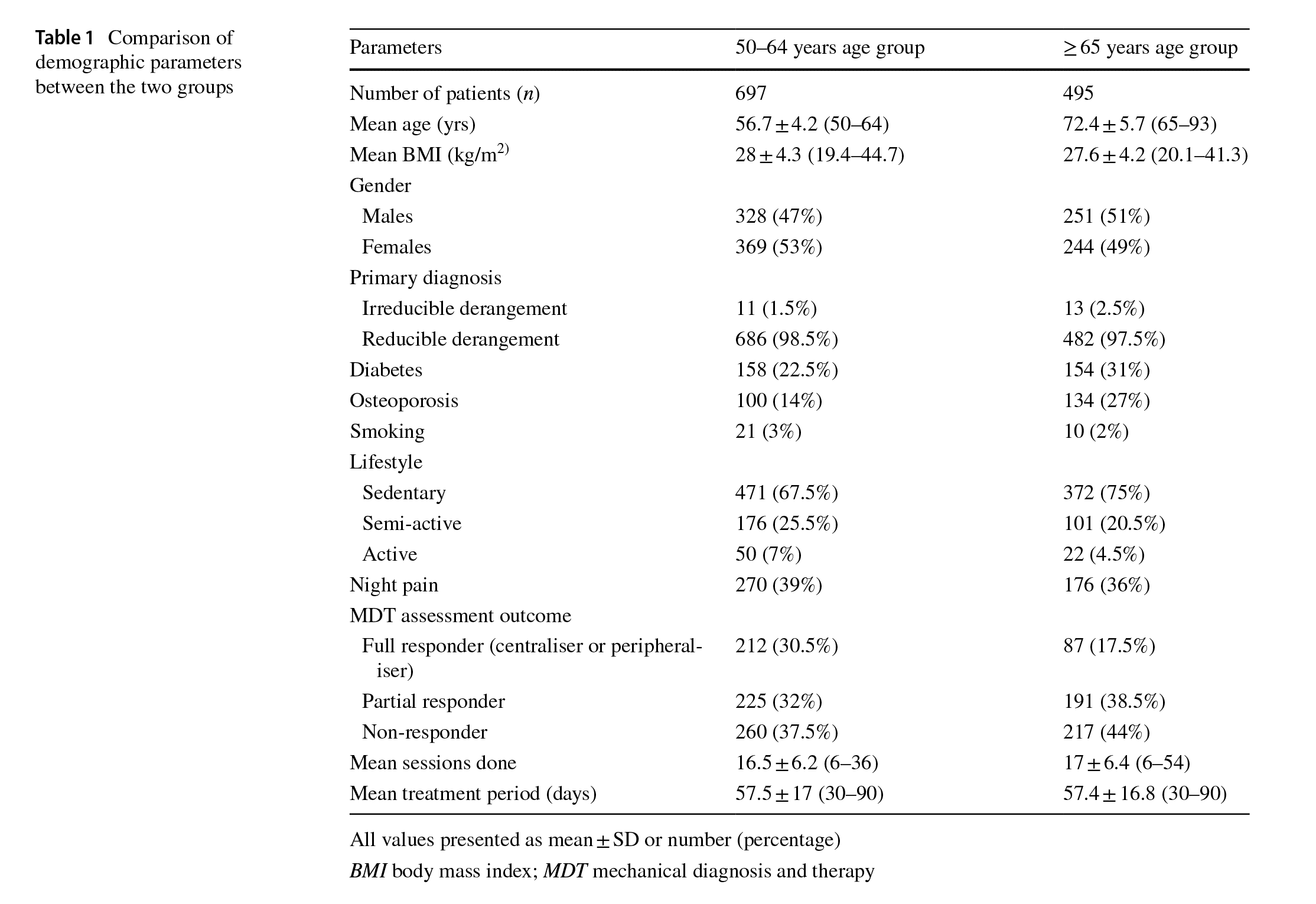
Based on the inclusion criteria, a total of 6330 patients with chronic LBP underwent physiotherapy at our centres out of which 1751 patients were in the 50–64 years age group and 1348 patients were in the≥65 years age group. Based on the exclusion criteria, 138 patients were excluded due to pathological conditions, 42 patients were excluded due to incomplete medical records and 874 patients were excluded based on the duration of treatment from the 50–64 years age group. Similarly, 212 patients were excluded due to pathological conditions, 57 patients were excluded due to incomplete medical records and 584 patients were excluded based on the duration of treatment from the≥65 years age group. Hence, data from 697 patients in the 50–64 years age group and 495 patients in the≥65 years age group were analysed for this study (Table 1). Both the≥65 years age and 50–64 years age groups were not signifcantly diferent in terms of gender (p =0.67),
BMI (p = 0.11), percentage of smokers (p = 1.00), percentage of patients with sedentary lifestyle (p = 0.27), diagnosis (p = 0.43), night pain (p = 0.27), duration of treatment (p = 0.91) and number of treatment sessions (p=0.17) (Table 1). However, the percentage of patients with diabetes was signifcantly higher (p=0.001), the percentage of patients with osteoporosis was significantly higher (p<0.001) and the percentage of full responders to movement testing was signifcantly lower (p<0.0001) in the≥65 years age group when compared to the 50–64 years age group (Table 1).
Before treatment, both groups were similar in terms of pretreatment mean NPRS score (p=0.38) and pre-treatment mean ODI score (p=0.11) (Table 2). Post-treatment, the mean NPRS score was signifcantly higher (p < 0.0001) and the mean ODI score was significantly higher (p<0.0001) in the ≥65 years age group when compared to the 50–64 years age group (Table 2). However, the mean percentage of NPRS improvement (p = 0.20), the posttreatment outcome categories (p = 0.06) and percentage
Multivariate analysis showed significant correlation between the post-treatment NPRS score and patient age (p = 0.024) and between the post-treatment NPRS score and pre-treatment NPRS score (p<0.0001) (Table 3). Similarly, post-treatment NPRS score improvement showed signifcant correlation between patient age (p = 0.002) and pre-treatment NPRS (p < 0.0001) (Table 4). Finally, post-treatment ODI score showed signifcant correlation between the incidence of osteoporosis (p=0.006) and pretreatment ODI score (p<0.0001) (Table 5).
This study aimed to determine the efcacy and clinical outcome of our integrated active, rehabilitation protocol in patients≥65 years of age with chronic LBP and compare the results in similar patients in the 50–64 years of age group with chronic LBP treated with the same rehabilitation protocol. We hypothesised that clinical outcomes with an integrated active, rehabilitation protocol for patients≥65 years of age presenting with chronic LBP will be similar to outcomes in 50–64 years patient age group. The results of our study indicate that after a mean treatment duration of 57 days, the mean NPRS score and mean ODI score achieved in patients in the 50–64 years of age group were signifcantly better when compared to the≥65 years age group. However, mean percentage improvement in NPRS score, post-treatment outcome category achieved and diference between the percentage of patients who achieved MCID thresholds for
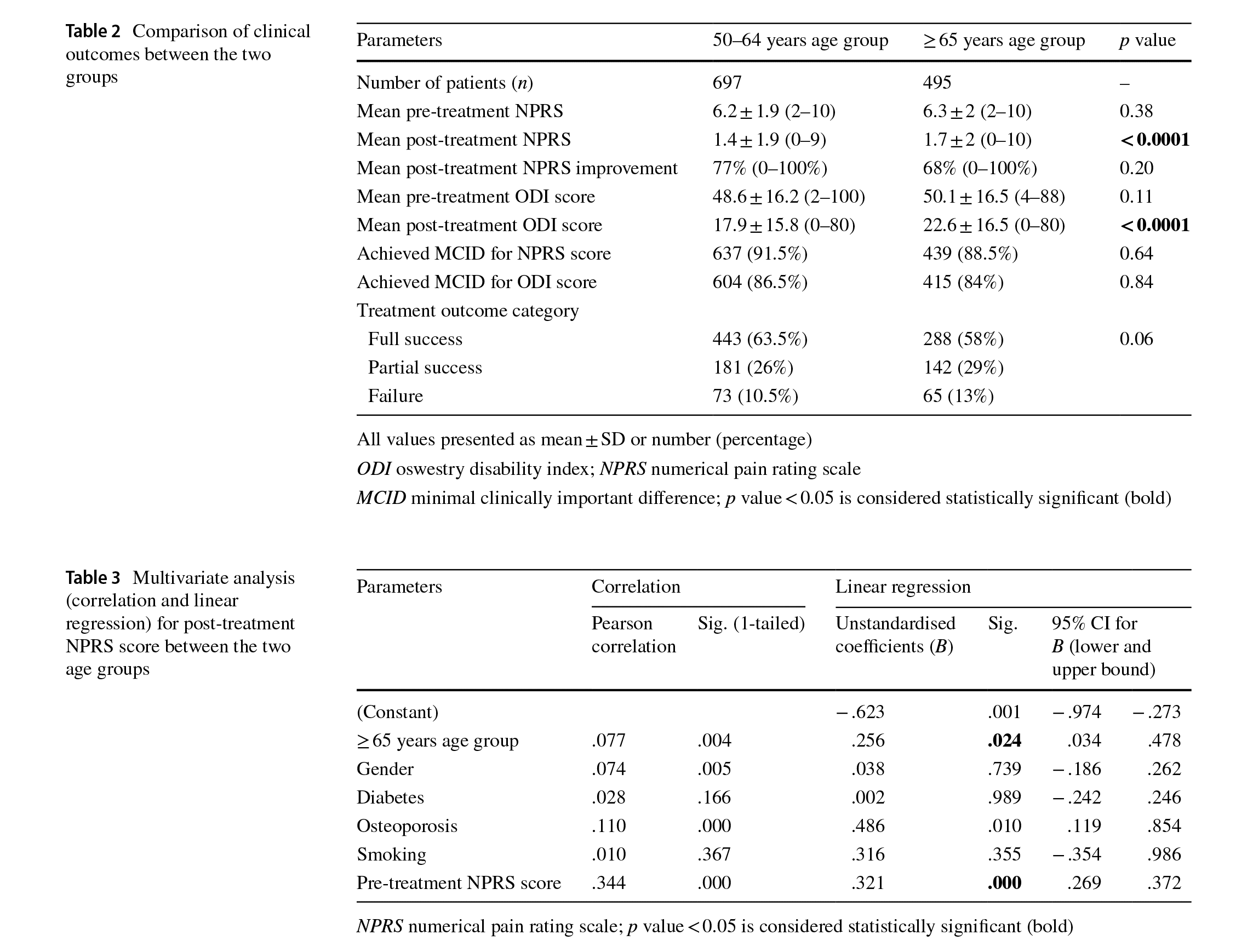
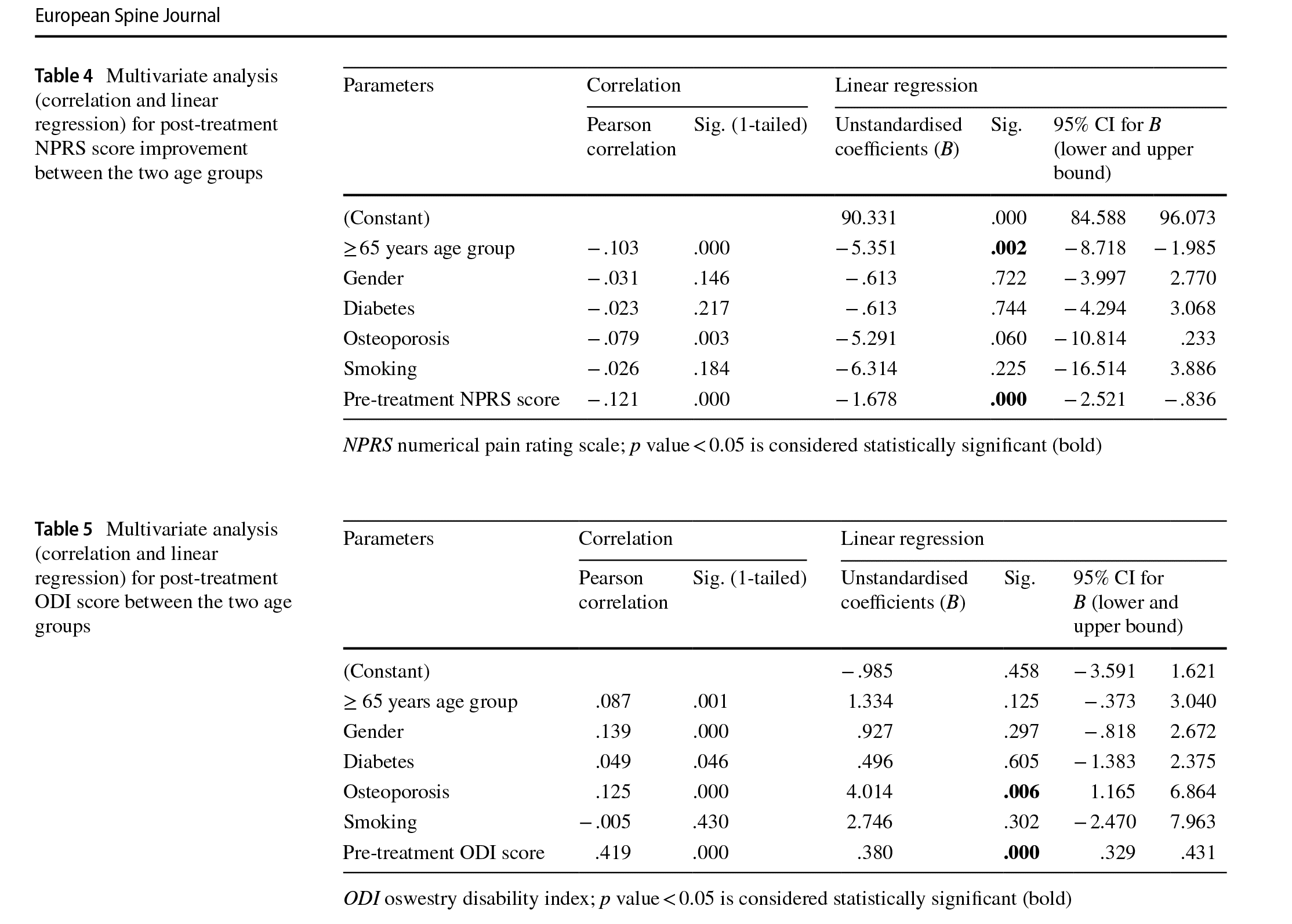
NPRS and ODI scores in patients in the≥65 years of age group were similar to the 50–64 years age group. Furthermore, post-treatment NPRS score and NPRS score improvement was signifcantly afected by the patient’s age and pretreatment NPRS score whereas post-treatment ODI score was signifcantly afected by the patient’s pre-treatment ODI score and incidence of osteoporosis.
In the literature, most studies evaluating clinical outcome of conservative management of chronic LBP in older adults involve varied treatment modalities such as oral pain medications, manual or manipulation therapy, acupuncture, tai chi, strength training, endurance training or a combination of various modalities [17, 29–31]. Beissner et al. [32] in an observational study of 69 elderly patients with chronic LBP treated with a community-based exercise and cognitive therapy reported improved outcomes for Roland Morris Disability Questionnaire (RMDQ), pain intensity at the end of 8 weeks. Hicks et al. [33] in an observational cohort study of 392 patients in ≥ 50 years age group treated with a community-based group exercise program (including spinal extension, abdominal strengthening and fexibility exercises) reported that 60.4% patients had improved back pain at the end of 12 months.
Similarly, Vincent et al. [34] reported signifcant improvement in pain intensity, disability and pain catastrophising after 4 months of resistance training in elderly patients treated for chronic LBP. These fndings were inferior to the outcome fndings of the current study in the≥65 years of age group where a mean 68% improvement in NPRS score was seen and 80.5% of patients had a NPRS score of≤3 at the end of treatment. This could be due to the fact that the above studies were conducted on a much smaller study population and primarily employed a communitybased group exercise program for treatment. Efective pain management and initiating exercise therapy early in the course of treatment for LBP is important to achieve good clinical outcomes and to ensure short-to-mediumterm benefcial efect of treatment [11, 35–37]. Hence, in our rehabilitation protocol, the frst step was to categorise patients based on pain intensity and to efectively lower pain intensity to NPRS score≤3 during the frst 2 weeks of treatment by using a combination of patient education, adjuvant microcurrent application and physical therapy interventions (low intensity active mobilisation exercises and directional movements) so that strengthening and stabilisation exercises can be initiated as soon as possible.
Our integrated, active rehabilitation protocol resulted in signifcant improvement in overall pain and disability status in the≥65 years of age group similar to clinical outcomes in 50–64 years age group. However, the mean NPRS score and the mean ODI score were signifcantly higher in the≥65 years age group when compared to the 50–64 years age group. This diference may be due to the fact that a signifcantly higher percentage of patients in the ≥ 65 years of age group had diabetes, osteoporosis and non-response to movement testing which may have resulted in relatively higher mean NPRS and ODI scores in the ≥ 65 years of age group. However, this statistically signifcant diference in the mean post-treatment NPRS and ODI scores between the two groups may not be clinically signifcant since the diference between the mean NPRS score between the two groups was 0.3 points and the diference between the mean ODI score between the two groups was 4.7 points (Table 2). Furthermore, both groups were similar in achieving MCID thresholds for NPRS and ODI scores.
Multivariate analysis showed that age, pre-treatment NPRS and ODI scores and the presence of osteoporosis had a signifcant efect on post-treatment NPRS and ODI scores. These fndings are similar to studies in the literature where baseline pain and disability have been reported to be important prognostic factors for recovery or functional improvement in patients treated with non-specifc LBP [35–37]. To the best of our knowledge, this is the frst and largest study in the literature which reported outcomes of conservative management of chronic LBP in patients≥65 years of age with an integrated, active rehabilitation protocol (Fig. 1).
There are some limitations to this study. First, posttreatment clinical data between 1 and 3 months was used to evaluate outcomes in patients for this study and long-term follow-up data were not used. The purpose of excluding patient outcome data of less than 1 month post-treatment was because most studies in the literature report short-term clinical outcome data at the end of 1 month post-treatment [38], and patient outcome data of more than 3 months post-treatment were excluded to minimise the possibility that patients may have improved with time than due to our rehabilitation protocol. Second, the elderly patient group (≥ 65 years of age) was compared with a similar group of patients in 50–64 years age group to determine the efcacy of our integrated, active rehabilitation protocol for the elderly. Ideally, the elderly group should have been compared with a control group of elderly where a diferent rehabilitation approach was used or where no treatment was administered. However, the main purpose of our study was to determine whether our integrated, active rehabilitation protocol for the elderly results in clinical outcomes similar to the ones in patients in 50–64 years age group with chronic, mechanical LBP. Third, the retrospective design of this study had its own limitations and biases and this study should be replicated as a prospective, randomised case–control study to efective determine efcacy of our elderly rehabilitation protocol. Finally, other variables such as causes of barriers to recovery and associated psychological and cognitive factors commonly afecting rehabilitation outcomes in the elderly patient population [39] were not part of the current study and must be included in any future studies evaluation the efcacy of rehabilitation in the elderly population for chronic LBP
The results of our study indicate that at a mean treatment duration of 57 days, the mean NPRS score and mean ODI score achieved in patients in the 50–64 years of age group were signifcantly better when compared to the≥65 years age group. However, our integrated active, rehabilitation protocol helped achieve signifcant improvement in NPRS score, MCID thresholds for NPRS and ODI scores and treatment outcomes in patients≥65 years of age, similar to patients in the 50–64 years of age group, at the end of 3 months of treatment. Post-treatment NPRS scores may be signifcantly afected by pre-treatment NPRS and patient age whereas post-treatment ODI score may be significantly afected by pre-treatment ODI score and incidence of osteoporosis.
Funding No benefts or funds were received in support of this study by any of the authors. This article is original and has not been published before or currently submitted to any other journal.
The authors declare that they have no confict of interest.
Ethical approval This study has been approved by an Institutional Ethics Committee and has been performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Visit our nearest clinic for your first consultation